“Chemicals forever” are much more acidic than what was believed

New and more precise acidity can help make PFAs easier to follow.
The per- and polyfluoroalkyle (PFAS) substances are nicknamed “forever chemicals” partly because their acidity helps them linger in the environment.
Many of these toxic chemicals are strongly acidic, so they have easily lost protons and take a negative charge, which allows them to dissolve in water and propagate more easily.
New works show that some APFs are even more acidic than previous estimates – key information to predict their movement through the environment and their potential effects on human health.
The study, led by the University of Buffalointroduced a rigorous experimental approach to determine the acidity for 10 types of PFAS and three current breakdown products.
Published in the journal Enstable Sciences and Technology LettersThe team reported acid The dissociation constants, or PKA values, which were generally lower and sometimes much lower than the previous experimental results and the predictions of computer chemistry. For example, the PKA of Genx, a replacement of perfluorooctanoic acid (PFOA) used in teflon manufacturing, was about a thousand times lower than a previously reported measure.
The lower the PKA, the more likely a chemical is likely to give up a proton and exist in its loaded form.
“These results suggest that the previous measures have underestimated the acidity of the PFAS.
The more precise PKA measures help efforts to understand the behavior of APFs in the environment. The pka of a chemical could signify the difference as to the dissolution of water, leftover on the ground or to a biological membrane, or perhaps volatilizing in the air.
“If we want to understand how these chemicals concerning propagate, it is very important that we have a reliable method for the precise determination of their PKA values,” explains Diana Aga, Phd, director of Renew and Suny Distinguished Professor and Henry M. Woodburn Chair in the Ministry of the Chemistry UB.
The work was supported by the National Science Foundation and carried out in collaboration with Scott Simpson, PHD, professor and president of the Saint-Bonaventure Department of Chemistry, and researchers from the Environmental Evaluation Institute and Water Research.
Combine experiences with calculations
The PFAs are made of a very fluoridated and repairing tail and a leading group more loving water. Many of the most examined PFAs have a very acidic leading group, which makes them more likely to abandon a proton and exist in its loaded form.
The fact that a PFAS exists in its neutral or loaded form depends on the level of pH of their environment. This is where PKA comes into play. He indicates to scientists the level of pH to which a given pfas is equal to the flip from neutral to loaded, or vice versa.
But there was a lot of disagreement on the PKA measurements of certain PFAs, such as the PFOA, with different teams offering very different values. One of the reasons for this can be the glass used during their experiences.
“PFAS like to stick to the glass. “In other cases, too much organic solvent is used to put the PFAs into solution, which also biaises the PKA measurement.”
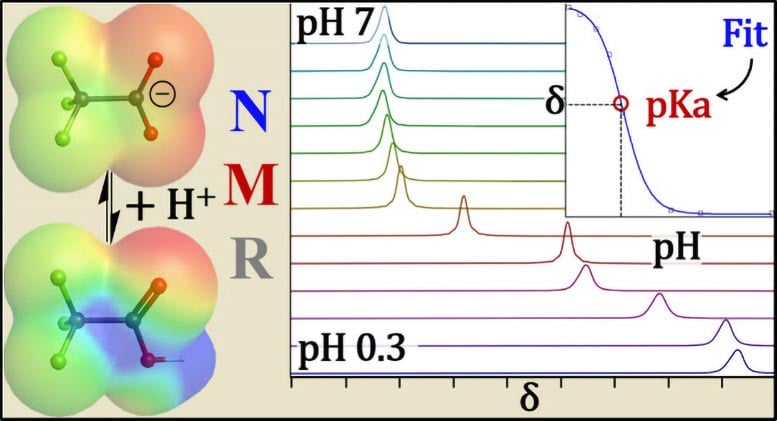
To take up this challenge, the UB team used spectroscopy of nuclear fluorine and proton nuclear resonance (hydrogen) – think of MRI for molecules. The RMN places a sample in a strong magnetic field and probe its atomic nuclei with radio waves.
When a PFAS head group is negatively loaded, neighboring fluorine atoms react to a different frequency (radio).
Reading these signatures at the atom level allows researchers to say if a PFAS molecule is loaded or neutral – capacities only other methods that have been used before cannot provide.
“This unique measure allows the RMN to intrinsically take into account the losses of PFAS in glass or other adsorption behaviors, so your PKA measurements do not end with the brand,” explains Hoepker.
Some PFAs are so acidic (pka of less zero) that generating them in their neutral form would require super-acid conditions (a level of pH lower than zero) which are not practical in standard laboratories. In these cases, the research team has associated RMN experiences with electronic structure calculations using the functional theory of density to predict the RMN offsets of neutral and ionized forms.
“We have increased the partial RMN data sets with calculation predictions to reach more precise PKA values,” explains Hoepker. “This hybrid approach centered on RMN – the integration of experimental measures with calculation analyzes – improved our confidence in the results and, to our knowledge, has not previously been applied to the acidity of the PFAS.”
PFAS problem has measured more precisely
The PFAS which was most difficult to measure is the PFOA, formerly commonly used in non -stick pans and deemed dangerous by the Environmental Protection Agency last year.
The team found that its PKA was –0,27, which means that it will be negatively loaded with almost any level of realistic pH. Previous experimental studies had measured its PKA up to 3.8 and more often about 1, while the Cosmo-R and Opera calculation methods had determined its PKA at 0.24 and 0.34, respectively.
Trifluoroacetic acid (TFA) – A PFA emerging more and more detected in the waters around the world and probably transported in the atmosphere and deposited by rain – turned out to be much more acidic than previously, with a PKA of about 0.03. Previous estimates had 0.30 to 1.1.
In particular, the team has determined PKA’s values for several prominent emerging APFs which had never been measured, such as fluorotelomer 5: 3 carboxylic acid (5: 3 FTCA), and PFAS ethers like NFDHA and PFMPA which are more recent APFs, but are also likely to pose challenges because of their health effects.
“This new experimental approach to determining PKA values for PFAs will have large -scale applications, to be able to validate derived calculation values, in order to facilitate the development of automatic learning Models that can better predict the PKA values of newly discovered PFAS contaminants when reference standards are not available, “explains Aga.” In turn, knowledge of the PKA values of emerging APFs will allow researchers to develop analytical methods, correction technologies and risk assessment strategies more effectively. “”
Reference: “Experimental determination of the PKA for 10 pfas, mono-, di- and acid trifluoroacetics by 19F-RMN” by Damalka Balasuryia, Aina Queral-Beltran, Tristan Vick, Scott Simpson, Silvia Lacorte, Diana S. Aga and Alexander C. Hoepker, August 12, 2025, Enstable Sciences and Technology Letters.
DOI: 10.1021 / ACS.ESTLETT.5C00688
Financing: US National Science Foundation
Never miss a breakthrough: join the Scitechdaily newsletter.

:max_bytes(150000):strip_icc()/chest-pain-GettyImages-2159567747-b503a35072d849dd93eb16177bd2f088.jpg?w=390&resize=390,220&ssl=1)

:max_bytes(150000):strip_icc()/Health-GettyImages-2205828799-568e686420e149e0aadafd8c2fd9b08f.jpg?w=390&resize=390,220&ssl=1)
