The medication at common constipation is promising in stopping the decrease in the kidneys

A constipation medication has proven promising in the slowdown in kidney damage in patients with chronic kidney disease by improving intestinal and mitochondrial health.
Chronic kidney disease (CKD) is a widespread and serious medical condition. For many people, the gradual loss of kidney function ultimately requires regular dialysis to prevent kidney failure and maintain life. Although CKD poses a major global health challenge, no drug has yet been approved that can directly improve kidney performance.
A team led by Professor Takaaki Abe to the Graduate School of Medicine of the University of Tohoku revealed a surprising possibility. They tested a normally prescribed medication for constipation and found that it can help protect kidney health. For the first time, this medication (Lubiprostone) has shown an ability to slow down the decline in kidney function in patients with IRC.
“We have noticed that constipation is a symptom that often accompanies MCDs and has decided to study this bond more,” explains Abe. “Essentially, constipation disrupts the intestinal microbiota, which aggravates the renal function. By working upside down, we hypothesized that we could improve the kidney function by treating constipation. ”
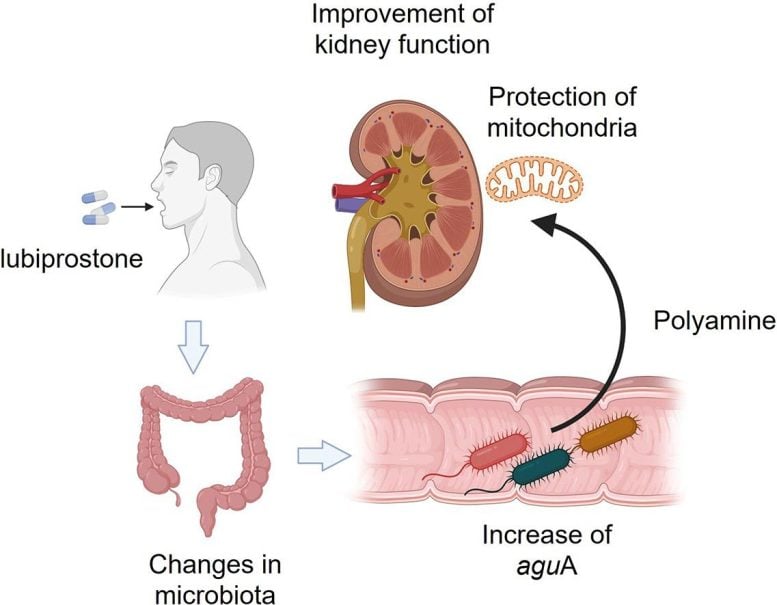
To explore this idea, the researchers launched a Phase II multicenter clinical clinical trial (Lubi-T trial) in nine medical establishments in Japan. The study involved 150 patients with moderate CKD and carefully assessed how lubiprostone influenced their kidney function.
Clinical trial results
The results have shown that, compared to the placebo group, the drop in renal function (defined as the estimated glomerular filtration rate: EGFR) has been deleted in a dose-dependent manner in patients treated with 8 µg or 16 µg of lubiprostone.
The researchers also studied the underlying mechanism in the way this effect occurred. They found that lubiprostone increases the production of spermidine, which improves mitochondrial function by promoting bacterial growth of the intestine. It has been found that the improved mitochondrial function exerts a Renoprotective effect – removing other renal lesions.
In the future, the research team plans to validate the results of the trial in a larger population (phase 3 clinical trial) and to advance the exploration of biomarkers who predict the efficiency of treatment. Their objective is to provide each CKD patient with the optimal treatment plan adapted to their needs. This discovery has the potential to significantly transform the conventional approach to the treatment of CKDs, which mainly focuses on the reduction of uremic toxins.
These results suggest a new therapeutic strategy in which laxatives remove the renal function. This strategy should contribute to the development of treatments not only for CKDs, but also mitochondrial dysfunction disorders.
Reference: “Lubiprostone in chronic kidney disease: overview of the mitochondrial function and polyamines from a randomized phase 2 clinical trial” by Shun Watanabe, Masaaki Nakayama, Takashi Yokoo, Satoru Sanada, Yoshifumi Ubara, Atsushi Komatsuda, Katsuhiko Asanuma, Yusuke Suzuki, Tsuhiko Asanuma, Yusuke Suzuki, Tsuheo Konta, Junichiro J. Kazuma, Takehiro Suzuki, Shinji Fukuda, Tomoyoshi Soga, Takuji Yamada, Sayaka Mizutani, Mitsuharu Matesumoto, Yuji Naito Taguchi, Kei Fukami, Hitomi Kashiwagi, Kenensi Suzuki, Hidetaka Tokuno, Marina Urasato, Ryota Kujirai, Yotaro Matsumoto, Yasutoshi Akiyama, Yoshihisa Tomioka, Shun Itai, Yoshiyasu Tongu, Eikan Mishima, Chiharu Kawabe, Tomoko Kasahara, Yoshiaki Ogata, Takafumi, Takafumi Toyohara, Takeya Sato, Tetsuhiro Tanaka, Takaaki Abe and Lubi -k -k Trial Investigators, August 29, 2025, Scientific advances.
Two: 10.1126 / SCIADV.ADW3934
Never miss a breakthrough: join the Scitechdaily newsletter.

:max_bytes(150000):strip_icc()/Health-GettyImages-1443971731-430faf537ff04d54bbce98f24e8e4b5c.jpg?w=390&resize=390,220&ssl=1)


